Last Friday Amarin Corp. plc (#AMRN) stock dropped nearly 20% after the company announced that they are raising $100 million through a "debt-like" financing and beginning to hire a sales force for the marketing of Vascepa. The stock price dropped because this move suggests that a buyout of the firm is not imminent. At Red Acre we have consistently reminded investors that a buyout now, after approval and before proven sales would be an exception and not the norm. We believe that Vascepa's New Chemical Entity (NCE) status is the next catalyst that investors will focus upon, and that if NCE status is denied, the stock has room to fall further as outlined herein.
Delay was for Financing
In retrospect, it is clear that AMRN's delay in deciding whether or not to hire a salesforce was most likely due to delays in arranging financing to shore up the balance sheet for a go-it-alone strategy. Had the company announced that they were hiring a salesforce without simultaneously announcing the non-dilutive financing, the stock price would likely have dropped much further. Since AMRN already had some $215 million in cash, adding $100 million in debt from Pharmakon Advisors ensures that they will be able to launch Vascepa for the Marine indication while continuing their clinical trials and post-marketing commitments.
According to their website, Pharmakon Advisors had $477 million under management as of April 2011. Lending nearly 21% of their Fund's cash under management to a single company is a bold move and a strong vote of confidence for AMRN management.
NCE Still in Play
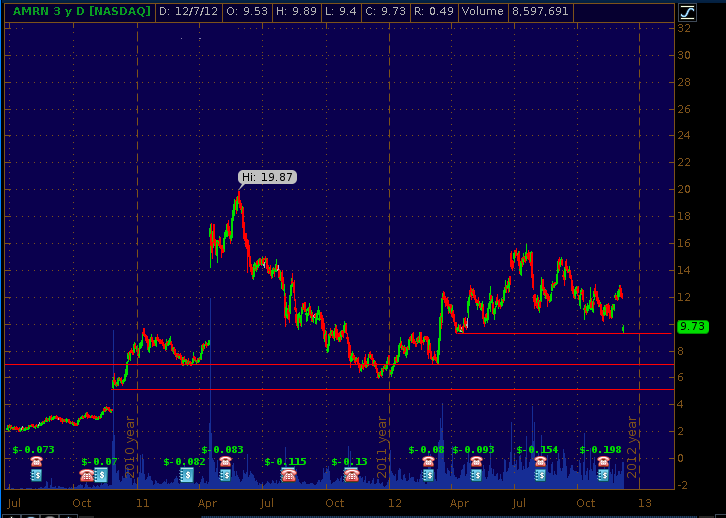
AMRN's stock price is currently finding support above the $9.30 level and could maintain a price above this support throughout this week in the absence of new information. While AMRN has announced the hiring of a sales force, the market appears to still be awaiting the NCE decision for Vascepa. The next FDA monthly update to the orange book is due on December 14th. If NCE status is granted to Vascepa, AMRN's stock should see a surge as buyout speculation will be renewed despite the company's decision to begin hiring a salesforce. If NCE status is denied or if no decision is rendered, AMRN's stock will test the $7 support and possibly even test the lows in the $5 range because lack of NCE status may cast doubt on an acquisition of AMRN within the next 12 months, after launch, but before the ANCHOR indication is approved. The 3-yr daily price chart below illustrates the support levels we see for AMRN.
Amarin Going it Alone - For Now
Despite management's continued insistence that they are still considering all three possible paths to commercializing Vascepa, investors would do well to focus on what management does rather than on what it says. Hiring of 250 - 300 sales representatives is a definitive step towards self commercialization. While management indicates that discussions with large pharmaceutical companies are on-going, clearly, these discussions have not met AMRN's expectations of an acquisition prior to commercial launch of Vascepa.
With time running out and no acceptable offer on the table, AMRN management is forced to prepare for self-commercialization in the Marine indication. The company is beginning with a sales force almost as large as Glaxo's sales force dedicated to Lovaza suggesting that they should be able to adequately address the patient population that falls under the Marine indication.
While some have criticized AMRN management for delaying the launch of Vascepa (the drug was approved more than 4 months ago), The delay is actually a net positive for the launch of the drug. Whether Vascepa is sold by AMRN, a partner, or an acquirer, ARMN's work in securing insurance coverage for the drug prior to commercial launch helps to ensure a healthy launch trajectory. The recent weak launch of Vivus' (#VVUS) drug Qsymia due to lack of insurance company coverage is an example of why rushing to market right after approval is not always the best course for a drug that relies on wide adoption to meet expected sales numbers.
Anchor Patents Pave the Way for a Future Buyout
The same day that AMRN announced the decision to hire a sales force and non-dilutive financing, AMRN's patent application number 13/272,520 covering the Anchor indication received "reasons for allowance" from the US Patent and Trademark Office (USPTO). This past weekend, another Anchor related patent, (application number 12/815,569), also received reasons for allowance. Reasons for allowance indicates that the patent examiner has finished the review of the application and prior art and has determined that the invention has novel claims. The next step in the USPTO process is a Notice of Allowance. Judging by the company's actions on previous patents, it is likely that AMRN will issue press releases when the Notice of Allowance is granted for these key Anchor patents.
Until these patents received reasons for allowance, AMRN had no patent protection specifically for the Anchor patient population. This patent coverage is likely a key topic of any potential buyout negotiations. AMRN Management's goal is to obtain a fair price in any potential partnership or buyout transaction. A price based not only on the Marine indication in very high triglycerides, but also based on the Anchor indication amongst patients with triglycerides between 200 and 500 mg/dl. The market for the Anchor indication is 8 - 10X as large as the Marine indication; however, without proper patent protection for the Anchor indication, a potential buyer should be unwilling to pay based on this larger market.
While AMRN management stated in their Q3 earnings call that lack of a decision on the NCE status is affecting negotiations with potential partners, we believe that issuance of the '520 and '569 patents will help in closing any gap in valuation between buyers and seller as the intellectual property estate is strengthened. Nevertheless, we reiterate our position that a buyout for AMRN now, before proven sales, is highly unlikely. After 2 or 3 quarters of proven sales, and once the Anchor sNDA is filed, a PDUFA date set, and the '520 and '569 patents issued, buyout talks may become serious.
Conclusion
In our last article about AMRN, we stated that "in the event that the company announces a self-commercialization strategy, the stock price will decline below $9." This week investors should watch the $9.30 support level. If this support level is breached, the stock's next major support level is around $7 followed by $5. We believe that the pending FDA orange book update will prop up AMRN's stock price; however, if no decision is rendered on Friday, or if the decision is negative, AMRN's stock price should fall below $9. A positive NCE decision may lead to a short-term boost to the stock price as buyout speculation renews; however, as we outline herein, a buyout is much more likely in 9 to 12 months when the Anchor indication is close to being approved and AMRN's IP portfolio includes several Anchor related patents.