From the launch of the year with the J P Morgan global healthcare conference, to the approval of Kynamro for ISIS (which we wrote about here), to the colossal failure of Celsion's HEAT trial (we suggested beforehand CLSN was not a buy at $8.11), and the subsequent dilutive financing deals that worked against shareholder's interests which we covered here and here, to the BIO CEO & Investor 2013 conference which we live tweeted (you can follow us on twitter: @redacre), it has been a busy first quarter and there is still one month to go. March and April 2013 are so chock full of events for Biotech that we can't cover them all in one article. Below we discuss the biotech news and binary events we are watching in March.
Arena Pharmaceuticals
March 4th Arena Pharmaceuticals (NASDAQ:ARNA) reports it's 2012 Q4 and full year earnings before the market opens. There is some speculation (unconfirmed rumors) that the company may have moved this conference call up by 1 week in order to coincide with the DEA's publication of the final schedule for BELVIQ. Should DEA publish the final scheduling decision and grant ARNA marketing partner Eisai Co. LTD's request to waive the 30 day waiting period, BELVIQ could become commercially available that day. Key for ARNA will be Eisai's execution of the launch roll-out, expansion of insurance coverage to ensure wider adoption of the drug and it's response to the European Union's day 180 List of Outstanding Issues. Expect the company to go before the EU's Committee for Human Medicinal Products (CHMP) in either April or May with final CHMP opinion issued during the June meeting.
ARNA's market cap is currently $1.8 Billion, which is $800 million greater than rival obesity drug maker VVUS. Recently announced wholesale pricing of BELVIQ at $199.50 per 1 month supply is at a significant premium compared to VVUS' Qsymia. While the bear thesis is that the efficacy of BELVIQ (on a placebo subtracted basis) is so low that the drug will fail, the bull thesis is that the premium pricing means that only on-label users (i.e. responders) will continue to use the drug, and their weight loss will be quite good. In clinical trials BELVIQ had 42% responders (those who lost at least 5% body weight by week 12). BELVIQ may experience high discontinuation rates as people try the drug and stop; however, the discontinuation rates may not be much different than those Qsymia is currently experiencing meaning that the premium pricing strategy might be the right approach.
While we are long ARNA we have scaled back our position to a very large extent on the recent run to the $11 range since the upcoming events around commercialization are not binary in nature and therefore do not fit our fund's investment style. Should the CHMP issue a favorable opinion on BELVIQ, a return to the $11 range is not out of the question with ARNA since rival VVUS is not a likely competitor in that market until their cardiovascular outcomes trial is completed; however, should the CHMP issue an unfavorable opinion, shares of ARNA have potentially $800 million in market cap to lose just to be on-par with VVUS. In the longer term, ARNA is positioned well to succeed in that it bears none of the marketing costs of BELVIQ, very little of the post-approval trial expenses, and has enough cash to focus on developing its pipeline. A drop in ARNA's share price due to slow rollout may be a buying opportunity for investors with a longer time horizon.
Depomed
March 4th Depomed (NASDAQ:DEPO) has it's FDA advisory committee meeting for Sefelsa - oral gabapentin, for treatment of vasomotor symptoms (VMS - or hot flashes). FDA briefing documents released yesterday did not give a clear indication of which way the agency is leaning. At issue is the fact that the clinical trials for Sefelsa failed to meet some of their primary efficacy endpoints with statistical significance; however, gabapentin is an already approved compound that is currently prescribed off-label for VMS. Further, the only other approved products for VMS are hormone therapies. An approval of gabapentin for this indication would mean that it is the first non-hormonal treatment available for a condition that affects millions of women for up to 5 year after menopause. DEPO has revenues from their approved products making the advisory committee vote an interesting binary play. If the committe decides to vote against the approval of Sefelsa and shares take a hit, this may be a buying opportunity. The March $7 Call/$6 Put straddle is relatively cheap given the binary event and could provide a payout regardless of which way the vote goes.
ImmunoCellular
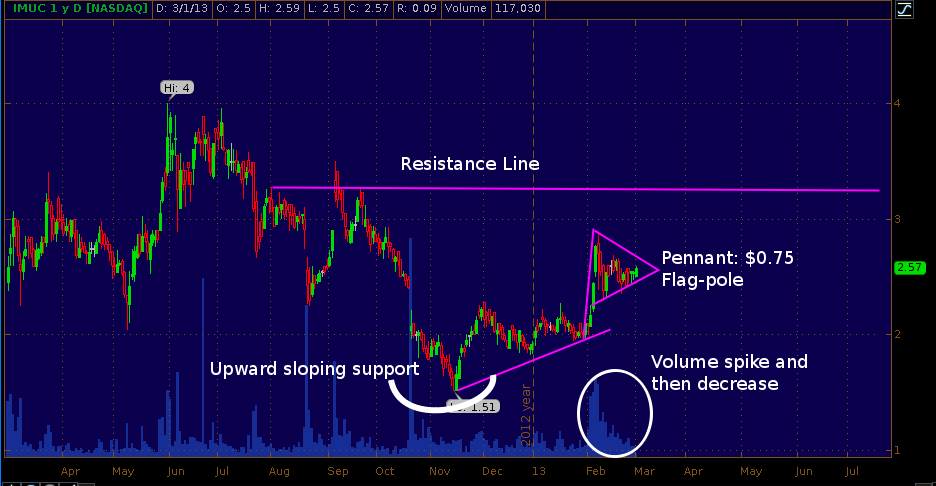
March 5th ImmunoCellular (NYSE:IMUC) reports 2012 Q4 and full year earnings. We traded IMUC entering a long position when the stock was in the $2 range and subsequently sold when the stock ran-up to the $2.75 range just 10 days later. Since then shares have been consolidating and the chart (see below) now makes a strong pennant formation. Investors are awaiting the expected Q1 announcement that the phase 2 trial of ICT-107 has reached its interim safety and futility analysis point.
ICT-107 is a cancer immunotherapy for glioblastoma multiforme (GBM) a deadly form of brain cancer. While the interim safety and futility check will NOT reveal any efficacy data, there is pent up excitement because the therapy showed promise in a (very small) single-center single-arm phase 1 trial. If the interim futility check announcement coincides with the earnings release (there is no reason to expect this since the two are actually unrelated), the pennant on the chart could see an upside breakout with room to move to the $3.25 range. IMUC is a largely retail investor driven stock as buysiders know that extrapolating from small single-center single-arm studies and making comparisons to historical trials is a very risky way to pick winning drug therapies. Furthermore, should IMUC produce successful phase 2 results later this year, the buy side will have a chance to buy in to the company when they raise cash to further their therapy towards commercialization. We remain neutral on IMUC as we are conducting additional due diligence on the entire cancer immunotherapy space and specifically on therapies for GBM.
IMUC one-year daily chart (click to enlarge)
Theravance
March 7th Theravance (NASDAQ:THRX) has an advisory committee meeting for RELVAR / BREO for the treatment of Chronic Obstructive Pulmonary Disease (COPD). We have no view on THRX as it is not currently in our coverage universe but 2 days before the meeting the FDA briefing documents will be released here. RELVAR is an inhaled dry-power formulation of a combination of a bronchodialator and an anti-inflamatory agent. Phase 3 results were promising and the drug is partnered with Glaxo Smith Kline (NYSE:GSK). THRX already has an approved product for treatment of skin infections and has another COPD drug under NDA review this year making for an interesting situation.
ISIS Pharmaceuticals
March 16th - 21st the American Association of Neurology has it's annual meeting. The event is of interest specifically for investors of ISIS Pharmaceuticals (NASDAQ:ISIS) because the company will be presenting data from it's phase 1 single-dose study of it's investigational drug ISIS-SMNRx for Spinal Muscular Neuropathy. The abstract for the presentation is available here. After the recent approval on Kynamro, investors have their eyes on the ISIS pipeline to see if the company will finally deliver on it's decades long promise of antisense therapeutics. The data from this phase 1 study are the next potential catalyst for ISIS. The company has 9 potential phase 2 or phase 3 data events due this year; although, we note that in the past, ISIS has been known to quietly kill off development of drug compounds without releasing negative trial data. After the approval of Kynamro, ISIS is poised to increase dramatically in value if a handful of these data events turn out positive.
A.P. Pharma
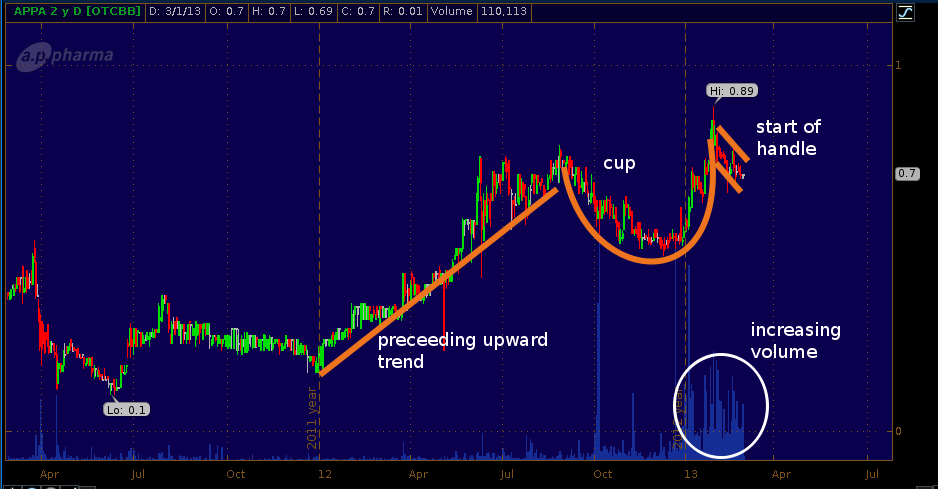
A.P. Pharma (OTC:APPA) has a PDUFA date in the last week of March for APF-530 which is their lead candidate being developed to treat chemotherapy induced nausea and vomiting. This is a second-round review of APF-530. APPA received a complete response letter (CRL) from FDA back in March 2010, citing CMC issues, concerns over the 2-syringe formulation/packaging of the drug, as well as requesting re-analysis of some of the phase 3 data and conducting some additional studies, in healthy volunteers, one for QT interval effects and two for bioavailability and metabolism of the drug. At the time, APPA was NASDAQ listed but after the CRL the stock price crashed and the company was de-listed becoming a bulletin board stock.
A look at the 2-year chart for APPA (see below) shows that the stock began running up from the 30-cent per share level at the beginning of 2012. The chart shows a cup and handle beginning to form. The dip in price and subsequent climb back up from mid-August to this past January, followed by the recent pullback form the cup and the beginning of the handle. Should APPA receive FDA approval, a continuation of the formation could occur.
Note, this is NOT a trading call on our part, merely an observation of the chart pattern. The company has some 350 million shares outstanding but the public float is only 139.8 million shares indicating that the capital structure needs to be examined more closely before trading the stock.
APPA 2-year daily chart (click to enlarge).
United Theraputics
United Theraputics (NASDAQ:UTHR) has a PDUFA date of March 31st for it's NDA resubmission for oral Treprostinil for treatment of pulmonary arterial hypertension (PAH). When UTHR announced FDA's acceptance of the NDA re-submission and that is was receiving a class-1 review (three month review), it caught the market by surprise. Back in October, when UTHR announced their CRL, the press release said that the FDA did not approve the NDA because:
The FDA letter questioned the clinical importance of the 6 Minute Walk Distance (6MWD) effect size shown in the FREEDOM-M study, the inability to demonstrate an improvement in time to clinical worsening in all three Phase III studies of oral treprostinil, and the inability to demonstrate a statistically significant effect on 6MWD in the two FREEDOM-C studies as reasons for being unable to approve the NDA in its current form. The FDA noted that it was unsure whether an additional clinical study could alter these impressions, but if United Therapeutics did undertake an additional study it should consider, among other things, a fixed dose design and more frequent dosing.
CEO Martin Rothblatt was quoted in the press release as saying:
We will continue using our best efforts to gain approval of oral treprostinil, and we will focus on doing so within the next four years.
Naturally the market did not expect the company to resubmit the NDA just 2 months later. Note that granting of a class-1 review of the resubmission means that no major changes were made. The FDA's guidleines for calssifying resubmissions indicate that class-1 review is appropriate for:
1) Final printed labeling 2) Draft Labeling 3) Safety updates in similar form to original safety data with changes highlighted 4) Stability updates to support product shelf life 5) Commitments to perform post-marketing studies and proposals for the same, 6) Assay Validation data, 7) Final release testing on the last 1 or 2 lots to support approval 8) Minor re-analysis of previously submitted data and 9) Other minor clarifying information.
How the company got from the FDA being unsure if a clinical trial would even convince them of the clinical utility of the oral version of the drug to making some minor changes and re-submitting is not exactly clear. During their latest conference call, UTHR's CEO suggested that based on the preponderance of evidence from previous clinical trials of Treprostinil (including the trials for the injectable forms), and based on the fact that the trials for the oral version of the drug "almost" reached statistical significance, the company felt that resubmission was warranted. Note that the company did have a face to face meeting with the FDA in December and the company believes that based on that meeting there was substantial agreement between the company and the Cardiovascular and Renal Drugs division of the FDA regarding the efficacy and clinical utility of the oral drug.
Treprostinil is already approved as an injectable drug and UTHR markets it under the trade name Remodulin. The drug generates over $450 million in sales for UTHR in injectable form, but is facing paragraph IV challenges from Sandoz who filed an abbreviated new drug application to make a generic version of Remodulin. UTHR is litigating the patent case, but approval of the oral formulation would give them a more preferred route of administration (oral vs. injection) and protect the business franchise.
At the present time we have no view on the likelihood of UTHR receiving FDA approval this month for oral Treprostinil.
Ziopharm
Ziopharm Oncology (NASDAQ:ZIOP) will be announcing top-line results from it's phase 3 trial of Palifosfamide plus Doxirubicin in Soft Tissue Sarcoma in the last week of March. Should the trial hit it's goal of showing a 3 month benefit in progression free survival with statistical significance, as well as a trend to overall survival benefit, the company plans to file for accelerated approval for palifosfamide in this indication. The company is at a critical stage and these trial results will be huge for them. We have done a fair bit of due diligence on ZIOP and will be publishing our insights on the company separately in the coming week or so (If you haven't already done so, signup using the form in the sidebar for the earliest notice when we publish it.)
Titan, Biogen-Idec
Later this month Titan Pharmaceuticals (OTC:TTNP) has an advisory committee meeting (we generaly do not trade and/or cover OTC stocks) fellow PropThink contributor Jason Napodano has been covering the name for a while. Here is Jason's most recent coverage of TTNP.
Other big news this month will be the PDUFA decision for BG-12 for Multiple Sclerosis from Biogen-Idec (NASDAQ:BIIB). BIIB is another company that is outside of our coverage universe, mainly because of it's size; however, BG-12 is an important drug for the company as it will be accretive to earnings if approved. The view on wall street is that the drug will probably be approved this month.