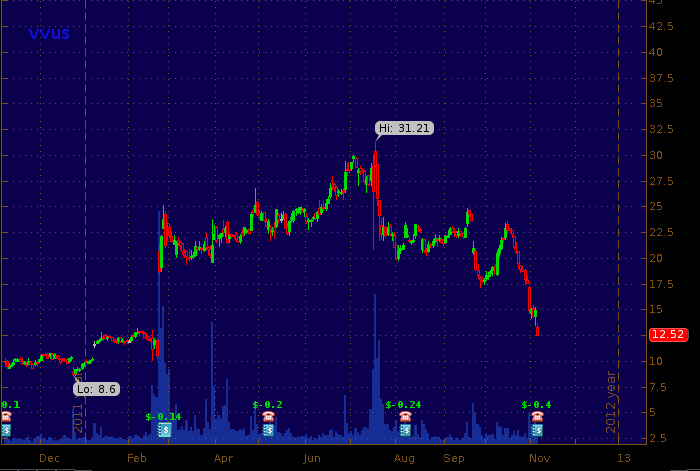
Obesity drug maker Vivus Pharmaceutical (#VVUS) released earnings and held their third quarter conference call this morning and the stock is selling off (down more than 20% as of this writing) over concerns regarding uptake of Qsymia and 3rd party payor coverage for the drug. VVUS is filling a large gap in it's price chart (see below) as investor enthusiasm is being tempered by management's remarks that indicate, in part, that they expect efforts to increase insurance coverage for obesity drugs to take 6 months to be reflected in sales numbers.
(data by TD Ameritrade)
Highlights from the Conference Call
On the conference call, the company stated that:
- To date 5,560 Qsymia prescriptions have been written to 3,504 unique patients
- 2,614 health care providers have written prescriptions for Qsymia
- 2,666 health care providers have undergone the REMS training
- Retail cost of Qsymia 30 day supply is $161.20
- VVUS is being reimbursed as Teir 3 with an average co-pay of $60
- 1 in 5 prescriptions are being covered by third party payors
- Prescriptions are being filled 1 to 2 weeks after being written
- 30% of prescriptions are being abandoned due to the cash outlay (because of lack of coverage)
- VVUS sales reps have made 28,000 details to the 25,000 doctors in their initial target market
The most troubling issues above are the 30% abandonment rate and the fact that only 1 in 5 prescriptions are being covered by insurers. The company stressed that they are treating the rollout as a new product category. As such, they are spending time educating providers about the disease condition, as well as the REMS program and how to prescribe the drug. The company has trained 200 speakers and have 400 speaking events planned for Q4 of this year.
Come Back in 6 Months
During the call, the company indicated that a lot of important events would occur "in 6 months".
- The company is appealing the EU CHMP rejection of Qsivia - the appeals process should take 6 months
- They have filed for modification of the REMS to allow marketing through retail pharmacies and expect to hear from FDA in 6 months
- Efforts to expand third party payor coverage should be reflected in sales in 6 months
In essence, the company was buying time and resetting expectations by suggesting that the slow launch of Qsymia will take about 6 months to reach any inflection point. Indeed, as they were wrapping up their prepared remarks, management stated:
We remain optimistic and excited about the future of the Qsymia brand and the medical obesity treatment category. We believe that progress on these key factors over the next 6 months will expedite early adoption, build a foundation for the new medical obesity treatment market and drive long-term Qsymia sales.
The market generally does not have the patience to wait 6 months for increased sales as reflected in the selloff today.
It is really too early to tell how Qsymia will ultimately perform in the marketplace. Clearly, the launch of the drug is going slower than many people expected. Lack of insurance coverage along with the additional burden of REMS and use of specialty mail-order pharmacies makes for a slow start.
VVUS made a tactical mistake by launching Qsymia in mid September. The company was aiming to gain traction by having a large presence at the Obesity Society annual meeting, but found itself in the awkward position of having to disclose a negative opinion trend from the EU CHMP at the same time that they were trying to drum up enthusiasm for Qsymia in the US. The launch timing also set up the present situation where analysts and the market are focused on just 6 weeks of data and are projecting future success or failure based on this initial data. Had the company waited until the beginning of Q4 to formally launch Qsymia, they could have effectively deferred questions regarding the sales rollout until the next quarterly conference call by which time their efforts to increase payor coverage would have been solidly underway. VVUS is transitioning from a development stage company, to one that is focused on commercializing a drug for a large audience. These types of transitions involve growing pains like the ones VVUS is experiencing regarding sales uptake.
The company has stated that they will not be giving out detailed prescription numbers in future conference calls, therefore, the company will be judged based on revenue instead. Management stated that as insurers pick up coverage for Qsymia, they will announce these advancements through press releases, promising several catalysts in the months to come.
Read Through for Arena
VVUS' launch experience with Qsymia has some implications for Arena Pharmaceuticals' (#ARNA) obesity drug BELVIQ. First, investors should temper their expectations for widespread adoption until insurance coverage is in place. To the extent that VVUS is educating payors about the obesity category in general, ARNA benefits from VVUS' efforts.
Similar to VVUS, ARNA and their marketing partner Eisai Co. Ltd. (#ESALY) will likely have to deal with the abandoned prescriptions issue for those who have to pay out of pocket; however this may be mitigated somewhat by two factors. First, since BELVIQ is awaiting DEA scheduling, Eisai has the opportunity to begin approaching insurers to expand coverage prior to launch. Watch the Eisai Q3 conference call for details on whether or not they are in fact using the time delay to gain coverage for BELVIQ. Second, ARNA should have an easier time in getting doctors to prescribe the medication. For a busy MD and staff, learning the REMS requirements for Qsymia and working through how to order the drug through mail order is something that takes an investment of time. In comparison, BELVIQ does not require any new procedures, and, if Eisai offers samples, the uptake of BELVIQ may be better than Qsymia because by the time patients have to pay for it, they will already have experienced the benefits of the drug.
In general, investors' expectations for Both Qsymia and Belviq have been very high, and ARNA has traded down along with VVUS over the past few trading sessions as the actual Qsymia prescription numbers have come in. This sympathy trading for ARNA should lead to more realistic expectations by the time BELVIQ is actually launched.
Conclusion
Our view is that VVUS' Qsymia rollout is too early to be deemed a failure, but that analysts' expectations for the stock will have to be reined in as the uptake of the drug lags behind projections. If VVUS does not reach an inflection point in sales by mid 2013, concerns over the cash burn rate and the need for additional financing will arise as expenses for the required cardio-vascular outcomes trial add to marketing expenses. For the moment, the market is discounting the future prospects for avanafil since the company does not have the resources to launch two drugs at once on its own. The thinking seems to be that if there was truly strong industry interest in partnering avanafil, negotiations would have resulted in a deal in the six months since the avanafil's approval. VVUS has a potential catalyst in the form of avanafil's EU approval sometime in the first half of 2013; however, the market's focus will remain on Qsymia and it's sales prospects for the coming months. The current negative sentiment for VVUS is likely to continue until significant progress is made on expanding payor coverage.
ARNA and Eisai may potentially have better payor coverage for BELVIQ at launch because the delay caused by DEA scheduling gives them time to approach payors prior to launching the drug. While payors are making decisions on coverage for the obesity category in general, it makes sense for them to look at Qsymia and Belviq at the same time. Furthermore, because BELVIQ dose not have the REMS requirements and limited distribution of Qsymia, an additional barrier to sales uptake is eliminated. Investors should keep in mind however that if payor coverage is not in place, ARNA may suffer a fate similar to VVUS a few months from now. Look to the ARNA conference call later today for further details from the company regarding launch preparations.